Last time we talked about the physics and chemistry of pigments, both natural and synthetic, and how they come to have the colors that we value. This time we’re going to discuss natural earth and mineral pigments, their origins, and their mineral and chemical composition.

Natural earth pigments are, according to archaeologists, some of the earliest pigments used for art by humans. The earliest paintings known included paints made from earth pigments, and charcoal. Earth pigments are, in their simplest form, soil that lacks organic matter, composed of mixtures of minerals, some of which confer color to the mixture. Soil can be classified by its mineral composition and its particle size, with clay being much smaller than (for example) sand particles. Clays are generally formed in river beds, with the action of flowing water grinding the mineral particles down to tiny sizes. But massive clay deposits, relics of ancient water bodies, are found in rocks on dry land as well. The earliest artists used naturally occuring clay of varying colors as the basis for their pigments, as the fine grains of clay are best for making smooth, regular pigment powders that can be mixed with binders or dispersants to make paints.

Photo by “BlueBreezeWiki.” This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
The bulk of the material in earth pigments is not the actual mineral that confers the color. The deposits of colorful clay that ancient and modern artists use come from the erosion of rocks into tiny particles, and reflect the origin and chemical composition of the original rocks. For the most part, these are mostly varying degrees of black or white or even crystalline and clear. Take a look at your beach sand. If your beach sand comes from the erosion of mixed continental rocks, you will see individual bits of clear quartz, or black or gray bits of other kinds of rock, mixed in with a few brightly colored grains of minerals. If your beach sand comes from fresh(ish) lava deposits, it could be very black. If your sand comes from coral reefs or limestone, it may be bright white. The famous pink sand beaches in Bermuda are mostly pure white grains of calcium carbonate from coral reefs, with a few bright red shells of a particular foraminiferan (a microorganism that makes a red calcium carbonate shell). If you were to clean and dry and grind Bermuda sand to the consistency of clay, you would have a pale pink pigment powder. Most of the naturally occurring earth pigments are similar sorts of mixtures; a ground of silicate-containing mineral rocks of no particular color, with bright mineral additives. Some synthetic pigments are made this way; a colorless natural earth is doped with a brightly colored synthetic mineral, so that it behaves similarly to a natural earth when used in pigments.
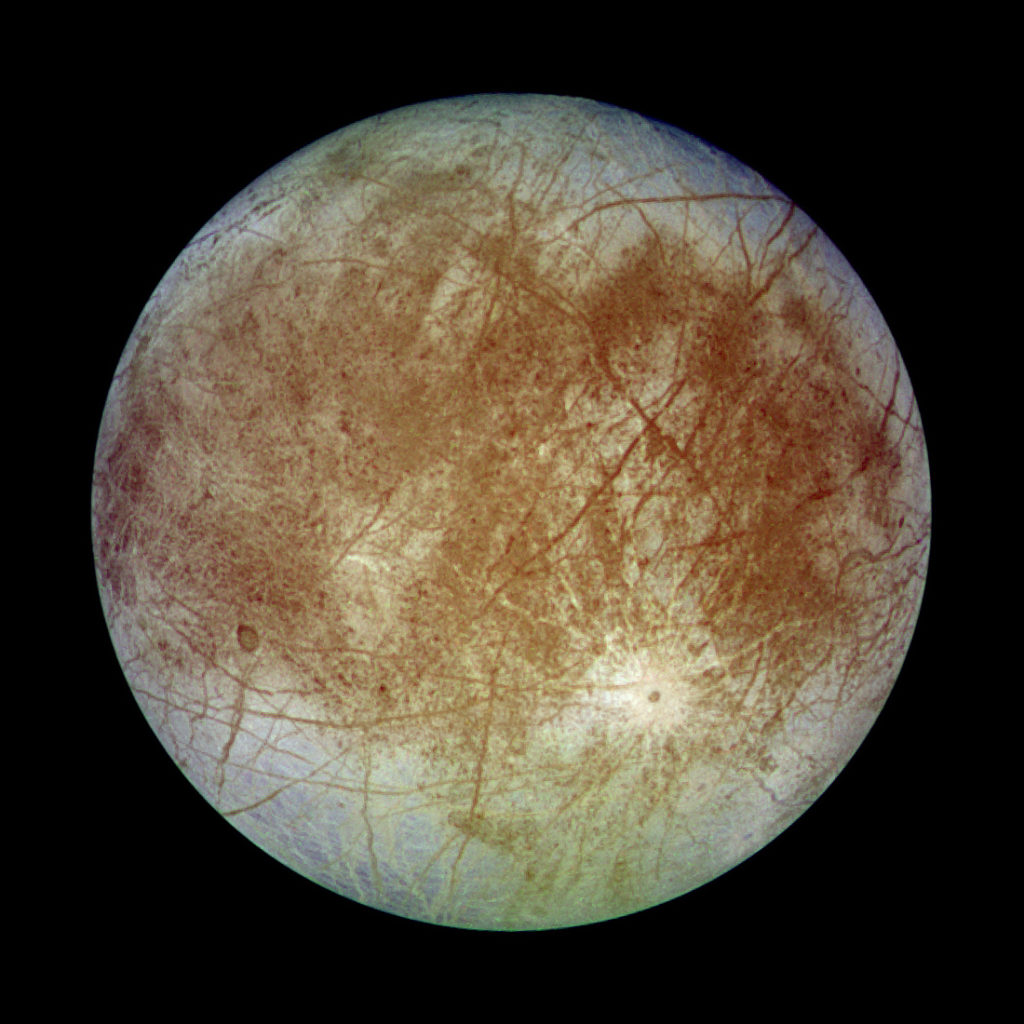
Future inventories of Ancient Earth Pigments may include Mars Ocher or Europa Red or even Pluto Violet. Photos: NASA
The most common and robust colors in natural earths are of course the reds and yellows and browns. These colors almost all come from various forms of iron oxide, complexed with water and oxygen in various ways that result in different shades, and mixed of course with the background material. Iron is quite abundant in the universe, as it is the heaviest element that can be made by the nuclear reactions of a normal star. To make larger elements requires a supernova explosion, and those are rather rare. So it is no surprise to see the familiar colors of rust and earth pigments in the canyons of Mars, the ice sheets of Europa, and the craggy surface of distant Pluto. Metallic iron, combined with water, leads to the familiar rust, which is one kind of iron oxide. Other iron oxides are formed through geological processes, involving heat or pressure or exposure to oxidixing chemicals other than water.

The mineral that gives yellow ocher its color is called limonite (not surprisingly). It is a hydrated iron oxide, which means the iron oxide (plain old rust) is complexed with water in a more or less permanent way that changes the electronic structure of the metal to change its absorption spectrum. The red in natural pigments often comes from hematite, which is an anhydrous (lacking water) iron oxide. Some hematites look purple (as in the sand at Pfeiffer Beach, in Big Sur), but it is the same mineral, and a feature of the grain size causes the red to have a purplish tone. Grinding the sand down to clay size may, sadly, eliminate the purple. Green colors in some natural earths come from iron in a different oxidation state, complexed with magnesium and aluminum, in minerals like celedonite (also, not surprisingly named).
Historically, natural clays found in the landscape were used by artists for pigments, giving each their local flavor. When trade began moving materials long distances across the Earth, pigments went with them. Desirable colors found in deposits in particular locations the world have become standards. For example, clay deposits found near the city of Siena or the region of Umbria in Italy have given their names to the colors that derive from their pigment powders. Sienna and Umber pigments have more manganese oxides than ocher pigments, leading to darker colors. Heating sienna and umber pigments causes some of the iron oxides to dehydrate, further darkening their color and leading to burnt sienna or burnt umber.
Naturally occuring colored clay is not the only way to obtain a natural mineral pigment, however. Certain minerals that occur in interesting colors and in large enough deposits can be ground finely to make a pigment directly. These minerals generally originate from infrequent and inconveniently located geological processes, deep in the earth. Making them available for use on the surface requires uplift of the regions where they occurred, and erosion to expose them, and perhaps even mining into the earth to find them. Frequently these situations occur in the high mountains of the world, where uplift and erosion can be ongoing processes in the present.
Lazurite photo by Didier Descouens: This file is licensed under the Creative Commons Attribution-Share Alike 4.0 International license.
Io photo from the New Horizons spacecraft (NASA)
The most precious pigment used in Europe in the ancient world and the middle ages – more expensive than gold! – was ultramarine, made from finely-ground bits of the semiprecious stone lapis lazuli. Lapis lazuli is a metamorphic rock (meaning, transformed by temperatures or pressures deep in the earth) found in several places around the world, but most importantly in the mountains of Afghanistan. Lapis lazuli from Afghanistan traveled very far in the ancient and prehistoric world, with ancient examples of lapis beads found in west Africa, and famously on the coffin of the pharoah Tutankhamen. The mineral that gives lapis lazuli its lovely blue color is called lazurite. The rock itself is often found with inclusions of pyrite (a shiny, gold or brassy iron mineral). The blue itself comes from a particular ionic state of sulfur. Burning sulfur in an oxygen atmosphere can produce blue flames (don’t do this). Another place you can see blue sulfur is erupting from the famous sulfur volcanoes of Jupiter’s moon Io. Iozurite, a blue pigment similar to lapis lazuli, may be a very precious AEP product of the far future.

Photo by Robert Lavinsky. This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
As you can imagine, from ancient times people have ground up interesting colored rocks and minerals to try and make pigments (at this point I should caution you not to grind up the gorgeous blue-green serpentine rocks of central California as this could be hazardous; the fibrous minerals are similar to asbestos). The ancients were familiar with the bright green mineral malachite as a copper ore: heating malachite over a hot fire causes it to break down and release the copper metal. But they also ground malachite to make a green pigment. Malachite is found in the deposits of ancient limestone caves, where mineral rich water ran over the calcium carbonate, producing reactions that precipitated the mineral into a solid. Deposits of malachite in the Fertile Crescent supplied the ancient world with copper for making bronze, but malachite is found in many places around the world. Malachite sometimes occurs alongside azurite, also a copper mineral, which is a bright blue. Azurite was also used as a pigment (like ultramarine, it is rare and expensive). Turquoise is another mineral rock whose blue color comes from copper, and whose formation involves the weathering of rocks (igneous, rather than carbonate). Deposits of turquoise are found in many places and it is one of the most important gemstones worldwide. Turquoise can be ground to make pigment, but it is more precious than malachite so this was rare.
Of course, artists have never been limited in the colors they use by what they can dig up from the ground. Next time we’ll talk more about organic pigments, derived from biological materials, and their origins.
Thank you for joining us as we do Pandemic Projects, meant to keep you energized, curious and learning!





Good article. One of the items high on my bucket list is a visit to Roussilon! Imagine wandering amongst those amazing colors! They also offer classes in everything from painting buildings to fine art. How about a field trip? — Bjo
Sign me up! 😀
[…] Last time on the blog we discussed inorganic natural pigments: natural colors from mineral, geological sources. This time we will talk about natural organic (meaning, containing tetrahedral carbon, as opposed to inorganic carbonates or graphite or diamond) pigments, of plant and animal origin. […]
[…] or thermal decomposition of metal salts. Metal oxide pigments are the most common kind of natural earth pigment chromophores, but synthetic techniques have yielded many more kinds of coordination complexes which yield […]